6. Methods: single-molecule imaging and super-resolution microscopy
In our lab, we use a variety of single molecule techniques to study biomolecular interactions.
Single-molecule fluorescence spectroscopy and super resolution tracking
Single-molecule fluorescence spectroscopy (SMFS) allows us to study the interactions of relevant biological species with the use of Förster resonance energy transfer (FRET) combined with alternating laser excitation (ALEX). These SMFS techniques are applied using microscopies with different illumination schemes: total-internal reflection fluorescence (TIRF) microscopy, variable angle epifluorescence microscopy (VAEM) and confocal microscopy.
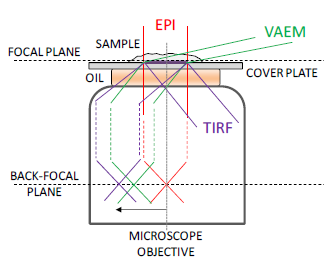
Near surface illumination techniques. Taken from (1).
The lab hosts a variety of microscope setups capable of different excitation and imaging schemes. The legacy setup in terms of SMFS is “HalfDome”, where two lasers (a 532-nm and a 638-nm laser) are used in TIRF configuration to allow for ALEX measurements of species on the surface of microscope coverslips. Using appropriate dichroic mirrors, the image obtained on a cooled EMCCD (ANDOR) is separated to two spectral channels which correspond to the emission of the two fluorophores in question, and allows for ALEX measurements.
A three-color ALEX/TIRF setup, known as “Headington Hill”, allows for 3 color ALEX excitation and imaging using lasers at 532, 641 and 730 nm. Read more...
Another setup, “Snowdon”, allows for three-color (lasers at 445, 532 and 638 nm) confocal measurements of ALEX excitation.
The work of PhD student Bo Jing led to the design of a new microscope, the Nanoimager. In an attempt to provide for a compact, portable, affordable and easy-to-use microscope for single-molecule fluorescence studies, Bo designed a microscope that retains the capabilities of the large custom-built setups, but offers them in a compact, fully enclosed box which makes it and more stable and laser-safe than its “big brothers”.
The first prototype, NIM1 (see image below), allows for 2 color ALEX/TIRF at 532/638 nm on an uncooled sCMOS camera, as well as HILO imaging and tracking of single molecule particles. A more advanced prototype, NIM2, has 3 lasers at 405nm, 532nm and 640nm, as well as a cooled sCMOS camera which allows a much larger field of view than the equivalent EMCCD cameras, as well as much higher frame rates, so more particles can be analysed from the same experiment with a better temporal resolution.
The success of the design led to the establishment of an Oxford University spin-out company, “Oxford Nanoimaging (ONI)” http://www.oxfordni.com, that is further developing and commercializing the device. The company is now selling their products worldwide. A commercial version of the Nanoimager featuring 405/532/640 nm lasers and high power (1 W) is placed in the MICRON imaging facility and is available to any MICRON user or visitor, including our lab.
Method development
A new method is needed in order to bridge the gap between the solution-based confocal measurements (limited in throughput and observation span due to its small volume, but with high temporal resolution), and the TIRF-based surface experiments (which allow for high-throughput but can suffer from the artefacts introduced by tethering to the surface). To answer this need, we are developing solution-based FRET methods which combines tracking and SMFS.
Another set up is currently being reconstitution-constructed to allow for 3D tracking of PALM/STORM particles, by utilizing astigmatism to map the depth into the 2D image.
Data Analysis
Custom software was written in order to address the needs of localization, tracking, FRET analysis and kinetic measurements. Different software was developed which allows for super-resolved localization of SM, FRET time traces and kinetics including analysis of three-color ALEX, super-resolved particle tracking and ALEX bursts in confocal movies. Further software is being developed to address single channel kinetics in TIRF experiments and FRET analysis in solution, as well as to improve the performance of existing software.
- Sinkó et al. Optics express. 2014, 18940-18948.








